We use cookies to ensure you get the best experience on our website. Learn more about our privacy policy.
Attention
This is an external link. Click “OK” to continue.
Antibe’s platform targets inflammation by harnessing the unique properties of hydrogen sulfide to improve the safety and potency of existing anti-inflammatory medicines. More than 20 years of academic and commercial research have uncovered hydrogen sulfide’s key role in a wide range of biological functions. These include the promotion of anti-oxidant responses, activation of anti-inflammatory genes and the unique ability to substitute for oxygen to produce cellular energy, underscoring its crucial role throughout biology.3,4,5
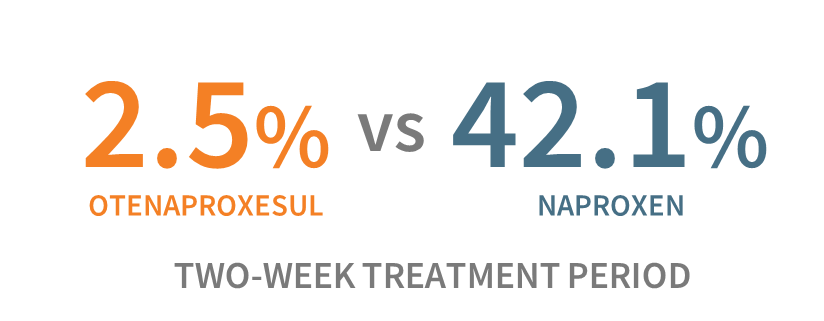
As the healthcare system responds to the opioid crisis, the market for acute pain therapeutics is evolving. NSAIDs, as the main alternative to opioids, are being employed more widely and have been shown to be equally effective in some acute pain settings.6,7 However, NSAIDs also triple the risk of serious gastrointestinal (“GI”) outcomes in a 14-day course of treatment.8 Antibe scientists have published Phase IIB data in the British Journal of Pharmacology indicating that naproxen, a widely used NSAID, is sixteen times more likely to cause gastric ulcers in a 14-day treatment period vs. otenaproxesul.9
Gastric Ulceration Rate 9
Commercialization
Otenaproxesul is licensed to three licensees for territories that include Canada, Israel and South Korea.
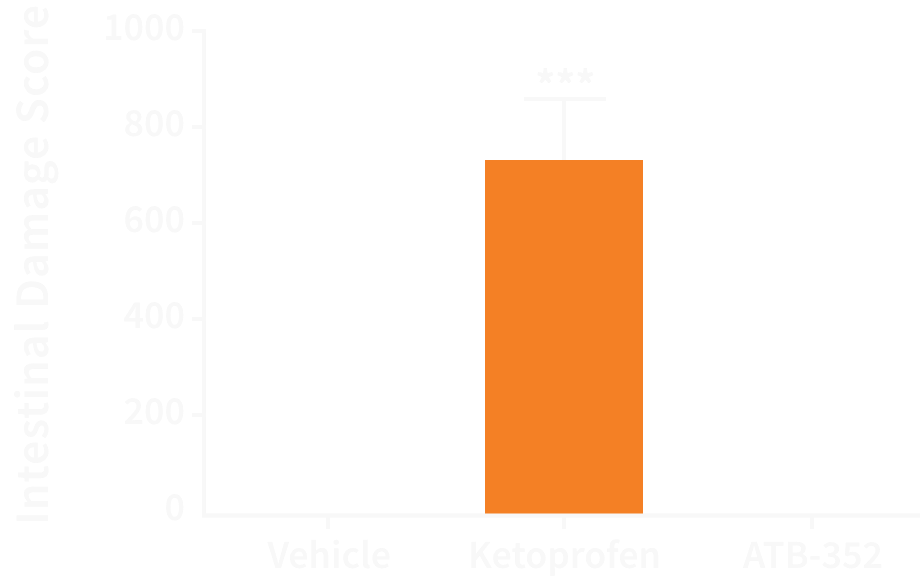
Our second pipeline drug, ATB-352, targets the need for a non-addictive analgesic for a specialized pain indication.
ATB-352 causes negligible GI damage in rats compared to ketoprofen, a strong NSAID used for a range of pain indications.
Targeting the need for a safer and more effective therapeutic for mild-to-moderate inflammatory bowel disease (“IBD”), the next drug planned for Antibe’s pipeline aims to help patients and prescribers delay the use of expensive, adverse effect-prone immunomodulators, steroids and biologics. Lead and back-up candidates have been identified for evaluation in animal efficacy models.

Antibe’s drug platform reflects more than two decades of research by our founder, Dr. John L. Wallace, along with his colleagues in academic and commercial labs around the world. The research program is overseen by Scientific and Clinical Advisory Boards, comprising fifteen internationally renowned scientists and clinicians, with specialties in pharmacology, gastroenterology and a range of inflammatory diseases. Antibe strives for the most rigorous level of scientific validation—clinical studies and trials for otenaproxesul have involved more than 800 subjects to date, including 384 patients in its Phase IIB dose ranging, efficacy trial.
Leveraging our drug platform to include gastrointestinal and aging-related conditions—pursuing our vision for improving health and quality of life for billions of people.
Antibe’s senior team is based in Toronto, Canada. We are supported by a global network of advisors and subject-matter experts covering scientific, medical, clinical and regulatory affairs, and business development.
……………………………………
1. Allied Market Research, Biotech Advisors, DataBridge, DelveInsight, GlobalData, Statista, Transparency Market Research, Antibe internal estimates.
2. GlobalData (2020).
3. Wallace JL and Wang R, Nature Rev Drug Discov. (2015) 14:329.
4. Zaorska E et al., Biomolecules (2020), 10, 323.
5. Olsen KR and Straub KD, (2016) Physiology 31: 60–72.
6. Lindbeck G, Prehosp Emerg Care. (2021 Dec) 20:1-17.
7. Choi et al., CMAJ 193 (24) (June 2021) E895-E905.
8. Fine M, Am J ManagCare. (2013);19(16 suppl):S267-S272.
9. Wallace JL et al., Br J Pharmacol (2019); 1-9.
10. U.S. Centers for Disease Control (2015).
We use cookies to ensure you get the best experience on our website. Learn more about our privacy policy.
